Contact UsCONTACT
Please feel free to contact us if you have any questions or concerns.
Inquiry FormStories
STORIES
SERIES EMBARK

AlphaNavi Pharma Inc. (AlphaNavi Pharma) was established in January 2019 as a carve-out venture from Dainippon Sumitomo Pharma Co. A carve-out is a method of combining outside investment, such as an investment fund, in addition to the mother company when a part of the business owned by the mother company is spun off as an independent company. In the case of Alphanavi Pharma, the research results of compounds developed by Dainippon Sumitomo Pharma for neuropathic pain, combined with the research results of pediatric limb pain attacks found at Kyoto University and Akita University, will be combined in April 2019 with the investment from Kyoto University Innovation Capital (Kyoto-iCAP), Dainippon Sumitomo Pharma and several venture capital firms raised approximately 900 million yen. We spoke with President and CEO Yoshihiro Oyamada and Chief Operating Officer Hirotsugu Hayashi about how the company was established and the status of its research and development. (Interviewer: Hidenori Takahashi)
Both Hayashi and I have been involved in pharmaceutical research and development for 20 years at Dainippon Sumitomo Pharma, and have been driving various development pipelines. DSP-2230 (current development code: ANP-230), a compound developed for the treatment of neuropathic pain.
Initially, it was treated as one of the promising development pipelines within DSP. However, around 2014, there was a change in strategy for DSP's focus areas. Dainippon Sumitomo Pharma decided to focus its research on neuropsychiatry, oncology, and regenerative and cellular medicine, and ANP-230 was given a lower priority.
After that, we explored various patterns of development methods within the company, and one of the options that emerged was to continue development in a carve-out venture.
I heard directly about the carve-out at the beginning of 2018. Even before that, I heard that talks had been in progress between DSP and Kyoto-iCAP. We considered options such as receiving investment from a major venture capital firm and continuing development in-house. It took about a year after we first heard about the project before we finally decided to carve out the project by combining the research results of Kyoto University and Akita University. Alphanavi Pharma was registered in January 2019, but it was not until April 2019 that we decided to receive outside investment. Both Hayashi and I remained employees of Dainippon Sumitomo Pharma until March 31, 2019.

Since there are few similar examples in Japan, we were not really aware of the existence of the venture itself. This is because there are very few examples of spin-outs or carve-outs from pharmaceutical companies when looking at domestic bio ventures. Most of them are university-originated ventures. In many cases, professors are participating in new companies while concurrently holding their current positions.
It is our passion for ANP-230. We are the ones who know the most about the characteristics and potential of this compound, which we have been researching and developing as project leaders. Although the probability of success for a pharmaceutical product is low, we had a strong feeling that ANP-230 could be launched on the market.
I have been involved in the research theme since around 2006. Later, I discovered ANP-230 in the course of promoting my research theme. I was involved in the construction of my own experimental system and evaluated it in animal models and electrophysiology experiments. Since joining Dainippon Sumitomo Pharma, I have been involved in dozens of research themes, and I believe that ANP-230 is one of the top three most promising compounds among them.
I was also very attracted to this compound and wanted to move it forward as much as possible. In talking with various parties toward the establishment of the company, venture capitalists were unexpectedly interested. Dainippon Sumitomo Pharma had the compound, and the venture capitalists were likely to provide the funds. Then the next question was what to do about the people. At that time, I thought that there might be areas in which I and Oyamada could help, and we decided to take a gamble.

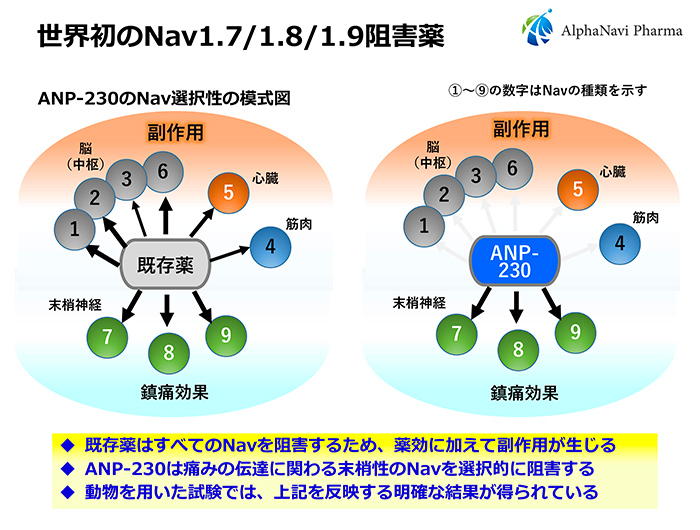
The target of ANP-230 is a protein called sodium channel (Nav), which is involved in pain neurotransmission. The most outstanding feature of ANP-230 is that it selectively inhibits only the Nav present in peripheral nerves. This opens up the possibility of oral drugs that could not be realized until now.
Nav is expressed in the human brain, heart, and peripheral nerves. Currently, about 10 types of Nav are known to exist, ranging from Nav1.1 to Nav1.9, and the distribution of Nav expression has also been clarified. There have been drugs that inhibit all Navs, from those in the brain to those in the peripheral nerves. However, if Nav expressed in the heart is inhibited, side effects such as bradycardia and arrhythmia, which can lead to death, will occur. Inhibition of Nav expressed in the brain can cause side effects such as dizziness and lightheadedness. In other words, there is no systemic drinking drug with a high safety profile.
Phase I has been completed at the global level at Dainippon Sumitomo Pharma: in the US and EU in 2014 and in Japan in 2016, respectively. The safety of the drug has been confirmed in about 200 healthy adult patients, and it has been confirmed that the drug does not cause cardiac or central nervous system side effects. The drug is safe and has a high potential to be an excellent treatment for neuropathic pain.
Dainippon Sumitomo Pharma was targeting a large market for diabetic neuropathic pain. If they had proceeded with clinical trials as is, they would have needed a large budget of several billion yen to bring the drug to market. Alphanavi Pharma would not be able to secure that kind of budget. Just as we were thinking about how to deal with the situation, we came across a disease called pediatric tetralimbic seizures through Kyoto-iCAP's intermediary. Pediatric Limb Pain Attacks is a new pain disorder discovered jointly by the group of Professor Emeritus Akio Koizumi of the Kyoto University Graduate School of Medicine (Alphavipharma's scientific and technical advisor) and the group of Professor Tsutomu Takahashi of the Akita University Graduate School of Medicine. The main cause of this disease was the overexcitation of nerves caused by mutations in Nav1.9. ANP-230 selectively and strongly inhibits the functions of Nav1.7, Nav1.8, and Nav1.9 in peripheral nerves.
Pediatric Limb Pain Attack Syndrome is a rare disease, so we thought that the number of subjects needed for clinical trials would be about several dozen. This is just barely large enough for a venture company to handle. We also believe that conducting a clinical trial of ANP-230, which has Nav1.9 inhibitory activity, in pediatric patients with tetralogy of fallopian tube syndrome, which is thought to be caused by overexcitation of nerves caused by Nav1.9 mutation, is a suitable clinical trial to prove the mechanism of action of ANP-230.
In November 2020, we submitted a Phase II clinical trial application to the Pharmaceuticals and Medical Devices Agency (PMDA), which was accepted. Our goal is to confirm the efficacy of the drug by the end of summer 2023. If we can achieve this, we will aim to expand the indication to Phase III for pediatric patients with tetralimbic seizures and other target diseases. Our goal is to bring ANP-230 to patients around the world who suffer from pain, and to make it widely available for peripheral neuropathic pain in general. Confirming the drug's efficacy in pediatric extremity pain paroxysms is only the gateway.
We are also considering developing another formulation using the same compound as ANP-230 as the active ingredient, one of which is a transdermal absorbent. We are calling the transdermal patch ANP-231. We have concluded MTAs (Material Transfer Agreements) with several companies and are asking them to consider the possibility of development.
Ultimately, we would like to proceed in the form of joint development with some other company. Some elderly people and young children have difficulty taking oral medication. A transdermal formulation would make it easier for such patients to use the drug.
There are both good and bad aspects. The good side is that we can proceed with research and development quickly based on our own decisions. In a large company, the work is divided into sections, so I think the process is that once we get to this point, we hand it over to the next section. Also, internal procedures take time. In a venture company, there are no such procedures, and we can make our own decisions, so it is very easy to do. On the negative side, it is difficult to carry out research and development and recruit human resources at the same time while working capital is not sufficient. We have a hard time planning for personnel because the timing of recruitment can be off if something unexpected happens, such as clinical trials not progressing as expected.
Before, we only had to look at the studies and projects we were responsible for. Now we have to do everything ourselves, and very often we have to make all our own decisions. On the other hand, now I can concentrate on ANP-230, think about the strategy, think about how to approach it, and work toward it. I think this is a very challenging and interesting part of the project.
Kyoto-iCAP took care of the initial capital policy preparation and intermediary services to the teachers. Even after the company was established, they have introduced us to various companies and consulted with us on licensing activities for the future. I talk with the outside directors dispatched by Kyoto-iCAP about once or twice a week.
(Interviewed in October 2021. Affiliations, positions, etc. are as of the time of the interview)
Research conducted by Kyoto University and Akita University has revealed that pediatric limb pain seizures are caused by activating mutations in the Nav1.9 gene. ANP-230, developed by Alphanavipharma, is the only drug in the world that suppresses Nav1.9 function. Ongoing clinical trials of ANP-230 in pediatric patients with extremity pain attacks are expected to advance our understanding of the pathophysiology of this condition. Kyoto-iCAP hopes that ANP-230 will become a new treatment option for patients suffering from a wide range of pain conditions, including pediatric extremity pain attacks.

Hiroyuki Ueno

AlphaNavi Pharma Inc. Website
Please feel free to contact us if you have any questions or concerns.
Inquiry Form