Contact UsCONTACT
Please feel free to contact us if you have any questions or concerns.
Inquiry FormStories
STORIES
SERIES EMBARK

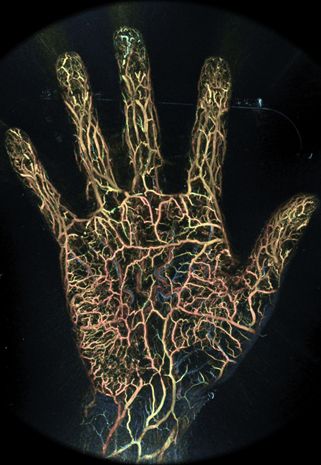
Microscopic blood vessels in the tips of the fingers and soles of the feet come to life on the screen. A new "photoacoustic imaging device" has appeared on the market that can visualize blood vessels at high resolution without the use of contrast media. It was developed by Luxonus, a start-up company from Kyoto University and Keio University. The innovative vascular visualization device based on photoacoustic technology was developed as the result of a joint research project between Kyoto University and Canon Inc. and was approved by the pharmaceutical affairs bodies as a medical device in September 2022. We interviewed Sadakazu Aiso, CEO of Luxonus, and Takayuki Yagi, CTO of Luxonus, who started the company based on the technology they have been researching for many years, about the R&D project, the start-up of the company, how the company came to market, and future developments. (Interviewer: Katsuyoshi Masuda)
It all started with the CK Project (Advanced Bioimaging Techno-Hub), a joint research project initiated in 2006 as an industry-academia collaboration between Kyoto University and Canon Inc. With the goal of creating innovation in the field of next-generation medical imaging that leads to the early detection of disease and the realization of a healthy society, we worked on the development of photoacoustic imaging technology that uses light and ultrasound to visualize blood vessels and other organs noninvasively. The CK project started when I was the director of Canon's Advanced Integration Laboratories, and I began developing the technology in 2008, and in 2014 I took over the development under the Cabinet Office's Innovative R&D Promotion Program (ImPACT), and as the program manager, I established a high-resolution 3D visualization technology using photoacoustic waves. I have been working on a project to establish a high-resolution real-time 3D visualization technology using photoacoustic waves and to demonstrate its clinical value.
The ImPACT program was in its final year of 2018, and Canon Inc. and Hitachi, Ltd. which had participated in the program ultimately decided not to commercialize the technology. I decided to start my own company after consulting with Dr. Aiso of the Keio University School of Medicine, who had participated in the ImPACT clinical research project.
Angiography using contrast media is used to visualize blood vessels, but it has not been possible to visualize minute vessels such as tumor vessels. In the field of plastic surgery, it is important to construct subcutaneous blood vessels in skin valvuloplasty, in which skin and fat tissue are transplanted and reconstructed from another area to a defect site, but there are large individual differences in subcutaneous blood vessels, and the inability to visualize subcutaneous vessels has been a major issue. The technology developed through the Kyoto University-Canon joint research and ImPACT can visualize such small blood vessels, and we believe it has high clinical value as it can be applied to a wide range of fields including plastic surgery, orthopedics, and ophthalmology.
Canon owned much of the original IP and was a key member of ImPACT, so it made sense for the company to commercialize the product. However, it was determined that the company would not commercialize the IP, and in addition, it was agreed that companies participating in the ImPACT program could use the ImPACT IP. Therefore, Luxonus was established in December 2018, the final year of the program, and participation in ImPACT made commercialization possible. Mr. Yagi joined Luxonus as CTO in April 2019, immediately after the program ended.

When I thought about starting a business, I went to consult with Dr. Nagahiro Minato, then Director of the Kyoto University Industry-Government-Academia Collaboration Division (now President of Kyoto University), who advised me to consult with Kyoto-iCAP because of its revolutionary technology in improving QOL (quality of life).
Meanwhile, I consulted with Keio University, which led me to the Keio Innovation Initiative (KII), and it was agreed that KII would provide support. The venture capitalists from both universities recognized the technological and social value of my company, and their cooperation, including their expertise in setting up a company, was a major factor in my decision to start my own business.
Furthermore, with the cooperation of the Japan Organization for Medical Devices Development (JOMDD), which has a proven track record in commercializing medical innovations centered on medical devices, we decided that we could start a business as a medical device manufacturer if we had the backing of three companies.

Photoacoustic 3D imaging technology involves two key devices: one is a near-infrared pulsed laser that emits two alternating wavelengths, and the other is an ultrasound sensor device consisting of 512 ultrasound sensors arranged on a bowl-shaped (hemispherical) curved surface. When a pulsed laser beam is irradiated onto the body surface, the red blood cells in the blood absorb the light, causing them to expand and contract. The ultrasonic waves generated by the expansion and contraction are detected. Since many red blood cells flow in blood vessels, ultrasonic waves are generated from various positions. Multiple sensors placed on the hemispheric surface detect them, and the position, running condition, and thickness of the blood vessels are calculated and imaged. This basic phenomenon also occurs on the surface of the body and in peripheral blood vessels in the hands and feet.
Using this technology, the photoacoustic imaging system can capture 3D images with a resolution of 0.2 mm over an area of 180 x 290 mm. In addition, 3D images can be displayed in real time for an area as small as 20 millimeters.
Traditionally, angiography and contrast CT scans were used to capture images of blood vessels smaller than 1 mm, which required the injection of a contrast agent. The use of contrast media carries the risk of complications such as contrast media allergy and kidney damage, as well as radiation exposure. The main feature of the photoacoustic imaging system is that it can visualize blood vessels safely and easily without the use of such contrast agents and without exposure to radiation.


We believe that the ability to visualize minute blood vessels is applicable to a variety of areas. For example, it is useful for preoperative planning for distinguishing between benign and malignant breast cancer, early identification of peripheral arterial disease, reconstructive surgery to restore function lost during surgery for breast cancer, head and neck cancer, and hand surgery.
Many diabetic complications, such as diabetic nephropathy, neuropathy, and retinopathy, are caused by hardening or blockage of small blood vessels. Peripheral blood vessels, such as the toes, may become hardened and clogged, causing ulcers and gangrene, which may necessitate amputation. Such severe cases can be prevented if the hardening and occlusion of peripheral blood vessels can be examined early with photoacoustic imaging devices.
For breast cancer, the tumor itself can be identified with contrast-enhanced MRI and ultrasound imaging equipment, but to determine whether the nature of the tumor is benign or malignant, we must rely on biopsy and cytology. Specimen collection may not be possible due to patient resistance, and there is a risk of progression and stage increase during follow-up with imaging diagnosis. We believe that the use of photoacoustic imaging devices has a high potential to lead to early diagnosis by imaging the vasculature of the tumor.
In reconstructive surgery, the importance of constructing subcutaneous vessels was pointed out, but accurate reconstruction and good prognosis can be expected if the location and thickness of subcutaneous vessels, which vary greatly from person to person, are determined in advance with 3D images and used for preoperative planning.
It can also be used to image tiny lymphatic vessels whose location has been difficult to determine. In lymphedema, which is caused by blocked lymphatic fluid flow, lymphatic vas deferens anastomosis is performed to eliminate the blocked lymph flow by bypassing the lymph vessels to the veins. Currently, it is difficult to accurately locate the micro lymphatic vessels and veins. Although contrast agents (ICG, indocyanine green) are used for imaging of lymphatic vessels, it is now possible to visualize the location of micro lymphatic vessels and veins, which will be useful for surgical planning.
In medical device manufacturing and marketing approval applications, it is necessary to pass a conformity survey to QMS standards, which are standards for manufacturing management and quality control, and a great deal of knowledge is essential for system development and operations. In our case, it was very significant that key members who had participated in ImPACT either changed jobs or were transferred to our company to take on these tasks. These members had experience in research and development, factory production, and quality control at the medical device manufacturer to which they belonged, and they had diverse knowledge and experience.
The reason why we were able to obtain approval in such a short period of time was that we applied for approval as a regulatory strategy, placing the highest priority on getting the product out to the public first. Specifically, we applied for approval as an improved medical device in the existing category of laser blood flowmeters, which allowed us to obtain approval without going through a lengthy clinical trial process.
Insurance coverage was approved on November 1, 2022, so we will first promote the product for use in blood flow measurement. The target areas are blood flow measurement of peripheral blood vessels in vascular surgery and microsurgery in plastic and orthopedic surgery, such as reconstructive surgery.
As the next step in regulatory development, we plan to conduct clinical trials and proceed to apply for and obtain approval as a diagnostic imaging device. The system can be used for diagnosis of breast cancer, peripheral arterial occlusive disease, and other diagnoses in the fields of breast surgery and vascular surgery. It will take time to demonstrate its usefulness through clinical trials, but if high reimbursement points can be expected, use in the diagnostic and medical checkup fields, where a large number of tests are performed, can be expected to increase sales volume.
On the other hand, in terms of expansion into overseas markets, we will promote global expansion into APAC, North America, and China. Overseas sales of medical devices require regulatory approval in each country and region, and we plan to expand after obtaining such approval. In addition, we have concluded a physical and chemical equipment agency agreement with China, Taiwan, Hong Kong, South Korea, and other countries by 2022 for physical and chemical equipment to be used in animal experiments for drug discovery.
I hope that those who aspire to start a business in the medical field are motivated by the need to help patients and contribute to the advancement of medical science. Modern medicine has achieved some of its goals, but we cannot be satisfied with the status quo and there are many issues that need to be resolved. For example, although cancer treatment has improved prognosis, it has not reached the point where QOL is restored. I hope that the resolution of such issues will motivate you to become an entrepreneur.
I believe there are three hurdles to starting a business in the medical field: first, it takes time to come to fruition, so it is important that the technology does not become obsolete; second, it is important to have a team and the prospect of funding for various tasks such as development, clinical research support, business licensing and approval; third, it is necessary to work with clinical doctors to research, develop, technology Third, we need to work with clinical doctors to research, develop, and nurture the technology. We will also need to be prepared to work steadily and collaboratively on these tasks.
(Interviewed in November 2022. Affiliations, positions, etc. are as of the time of the interview)
In Japan, there is a term called "device lag," meaning that there is a considerable delay in the practical application of medical devices compared to Europe and the United States. Luxonus has boldly taken on the challenge of developing new medical devices in Japan and has achieved regulatory approval and insurance coverage in less than four years since the company was established. This is due to the fact that Luxonus has assembled a team of medical device development professionals from academia and industry, including President Aiso and Director Yagi. With the approval in Japan, Luxonus aims to expand into overseas markets in the future.

Osami Kono

Luxonus Inc. Website
Please feel free to contact us if you have any questions or concerns.
Inquiry Form