Contact UsCONTACT
Please feel free to contact us if you have any questions or concerns.
Inquiry FormStories
STORIES
SERIES EMBARK

Retinitis pigmentosa is an inherited, progressive disease that causes abnormalities in the light-sensing retina in the eye. Night blindness and visual field narrowing occur gradually, progressing to loss of vision and color blindness, and may eventually lead to blindness. It is the second leading cause of blindness in Japan and is a designated intractable disease (rare disease) with an estimated 2 million patients worldwide. To address this unmet medical need for which there is currently no effective treatment, Restore Vision Inc., a start-up company from the Keio University School of Medicine, is taking on the challenge of developing a gene therapy drug that aims to regenerate vision.
The gene therapy developed through collaborative research between Keio University School of Medicine and Nagoya Institute of Technology, backed by the research tradition cultivated in the Department of Biophysics, Division of Biological Sciences, Graduate School of Science, Kyoto University, is based on a technology called optogenetics, in which nerve cells are genetically engineered to respond to light. The company has been in the process of developing a gene therapy for the regeneration of visual acuity, the world's first gene therapy for the regeneration of visual acuity. We interviewed Yusaku Katada, President and CEO, and Hikaru Miyazaki, COO, about the company's start-up process and business prospects as it aims to become the world's first company to realize a vision regeneration gene therapy.
(Interviewer: Katsuyoshi Masuda)
After graduating from Keio University School of Medicine, I went on to graduate school to study gene therapy in ophthalmology after gaining clinical experience in ophthalmology. Since high school, I had wanted to be involved in research that could help people around the world, and at the time, the human genome was being decoded and was a hot topic, and I was also interested in genetic engineering. I chose ophthalmology because I thought I could combine research and clinical practice, and because ophthalmology is leading the way in the application of advanced medicine, as the first area of regenerative medicine using iPS cells was in ophthalmology.
The new field of research we started in the ophthalmology laboratory was to try to regenerate vision by applying a technology called optogenetics, which uses light to control neural activity, as it was just beginning to flourish. That is what we are now targeting: vision regeneration gene therapy for retinitis pigmentosa, an intractable disease.
In optogenetics-based visual regeneration, photosensor proteins (photoreceptor proteins) that catch light and convert it into biological signals play an important role. A major issue with conventional optogenetics is that it cannot produce a level of light sensitivity that is practical for visual regeneration. When we were thinking about how to engineer a photosensor protein that could be used for gene therapy, we came across research results from the Nagoya Institute of Technology. When we consulted with Dr. Hideki Kandori, a professor at Nagoya Institute of Technology and a graduate of Kyoto University, we got the feeling that a chimeric protein of microbial rhodopsin and animal rhodopsin (chimerarhodopsin) could be used, and we started a collaborative research with the Kandori lab in 2015.

In fact, I myself had not thought about starting a business at all during our joint research. As a physician and researcher, I only wanted to solve social issues such as the living difficulties of patients and the burden on those around them caused by blindness, but Dr. Kazuo Tsubota, then professor in the Department of Ophthalmology, encouraged me to start a business. Dr. Tsubota had just launched his own venture company and was working on fostering a venture ecosystem that would lead to the current Keio University Medical School Venture Council, and he suggested that I start a business. This is the story of how I founded Restore Vision in November 2016, when I was still a graduate student.
D. in March 2019, but I was the only one in charge and was groping in the dark to build the organization. I had negotiated with various VC firms, but they did not take me up on the offer because I had no knowledge of fundraising, so I took advantage of the angel taxation system to obtain investment from professors related to university medical schools to fund operations.
Later, I met Teruki Miyazaki, who happened to attend an event of the Keio University Medical School Venture Council, and we hit it off and he joined Restoration Vision in March 2020.
Since my student days, I had always wanted to build a career in a job that would commercialize technologies from academia, and after working at the Development Bank of Japan, I served as COO and CFO of several venture companies. With the idea of taking on the next challenge, I was introduced to Kataida by a mutual acquaintance when I attended an event at the Keio University School of Medicine. I felt that we would get along well since we were the same age, and since Katada was a one-man company, I felt that there were many factors that I could use to my advantage. Of course, I understood the uniqueness and superiority of the company when I heard about its technology from Mr. Katada.
At the time, the talent that Katada was looking for was someone with experience in pharmaceutical company business. I was not eligible for the position, but I honestly informed them of my intention to join the company at the end of 2019 and joined as a sort of pushover wife.

In running a start-up company, do you want to be successful as a company, earn revenue, or gain prestige as a venture manager? There are many ways to think about it, but what we decided together at the beginning was to make it our top priority to deliver the drugs we developed to patients. We did not talk much about building a structure as a company organization.
For the first two years or so, there were two of us in full-time positions, and the rest of the development was carried out by people who participated in the project on a side or concurrent basis. Today, we have 10 full time positions, but we have more than the same number of part time, side hustle, and dual employment members running the business.
To begin with, people in Japan who have been involved in the development of gene therapy are extremely rare. It is very difficult to find such a person to join a weak venture on a full-time basis. We thought it would be easier for people with expertise to participate in the project in the form of a dual job, and as a result, the best way to proceed with the project was in its current form.
We were aware that drug discovery and pharmaceutical development requires people who can manage development progress in reverse based on the original goal and who have the experience to link this to global development. In order to benefit from the wisdom of such people, we have settled on a side job as a work style.
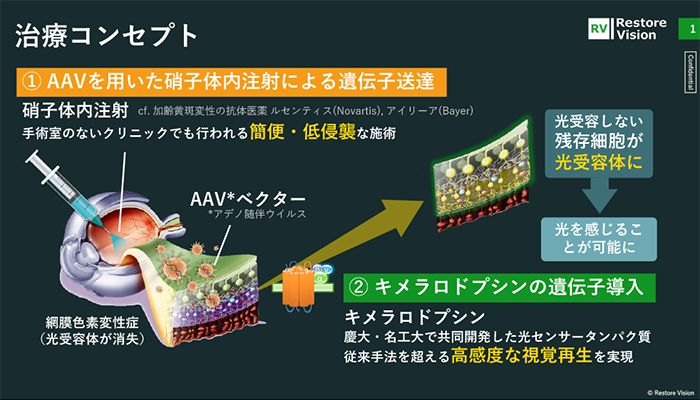
The goal is to realize a highly sensitive gene therapy for visual regeneration using chimerarhodopsin, a proprietary technology developed through joint research and development, which allows patients to see even at night. Chimerarhodopsin is a light sensor protein optimized for visual regeneration that combines the best features of animal and microbial rhodopsin.
There are several overseas ventures that are attempting to regenerate vision using optogenetics, but most conventional photosensor proteins use microbial rhodopsin, which responds to intense light such as sunlight, but cannot respond to light in everyday life. However, the problem was that they could not react to the light of everyday life. The technological advantage is that we have overcome this problem.
There are various techniques for delivering genes to cells, but our gene delivery process uses a gene carrier called an adeno-associated viral vector (AAV vector), which is also used in approved drugs. By injecting this AAV with a gene that expresses chimerarhodopsin into the vitreous body of the eye, a therapeutic agent is injected to express a light sensor protein that enables visual regeneration. Intravitreal injection is an administration method used in ophthalmology clinics as a treatment for various eye diseases such as diabetic retinopathy and age-related macular degeneration, and its second feature is that it is a "simple and minimally invasive" procedure.
In the non-clinical phase, we have been developing formulations for investigational drug manufacturing at contract manufacturers and conducting animal studies such as pharmacological and safety studies. We compiled those results and materials and submitted a notification of clinical trial to PMDA (Pharmaceuticals and Medical Devices Agency) in October 2024.
A Phase I/II clinical trial for patients with severe retinitis pigmentosa has just started at the investigational site, Keio University Hospital (press release issued by the company and Keio University School of Medicine on February 13, 2025).
Gene therapy drugs often target rare diseases, and the royal road is to aim for approval in the U.S. with a view to global development, but we are proceeding in parallel while leading the way in Japan. Based on the results of the clinical trials in Japan, we had no choice but to expand globally through the next round of funding. Fortunately, we were selected by AMED (Japan Agency for Medical Research and Development) for the FY2023 "Drug Discovery Venture Ecosystem Enhancement Project" and were able to proceed with preparations for global clinical trials in parallel with domestic trials. We were able to proceed with preparations for global clinical trials in parallel with domestic clinical trials.
The most difficult part was the development and manufacturing of investigational drugs, because just as it is extremely difficult to produce homogeneous, high-quality cells for regenerative medicine using iPS cells, it was also difficult to produce investigational drugs that could be administered to humans. I think this is one of the reasons why major pharmaceutical companies have not been able to get involved in gene therapy drugs. To begin with, the number of people involved in the development and manufacture of gene therapeutic drugs is limited worldwide, and when researchers from academia work in a venture company, it is very difficult to manage them. This is also an ongoing challenge.
We basically conduct R&D without a facility, so the breakthrough point for us was to find partners involved in drug development and manufacturing. Specifically, it is important how we can connect and collaborate with partners such as CROs (contract research organizations) and CDMOs (contract drug development and manufacturing organizations) and key persons in the industry. In particular, we experienced many failures in manufacturing, but we were fortunate that we were able to manufacture an investigational drug as a result. In addition, we were able to connect with key people in the industry, thanks to the results of various activities based on Katada's research activities, as well as the support of the lead VC, we were able to attract the interest of key people such as overseas doctors, researchers, and patient groups, and BD representatives from major global pharmaceutical companies. I think that's the point and our strength. I believe this is a key point and a strength of the project.
Kyoto-iCAP provided not only investment but also encouraging support through its connections with key people, including the introduction of a consultant with extensive experience in dealing with the PMDA, as well as the introduction of the current General Manager of the Business Administration Department, who had worked for a foreign pharmaceutical company for many years.

This may sound a bit clichéd, but I think it is important to break out of the egg (academia) shell and start up a company for now. I can't speak strongly about this, as I myself was pushed to do so.... However, when you become a manager, risk-taking becomes a major issue, so you need to be prepared for it.
In fact, at the beginning of my participation in the project, we both decided on the criteria for withdrawal after a year or so. Specifically, we decided that we would stop if we could not either secure funding or obtain a research grant of several tens of millions of yen. As a result, we were able to achieve both of these goals, but I think it is important to have a decision criterion for withdrawal. If we can make the decision to withdraw, we can take up the challenge again.
(Interviewed in February 2025. Affiliations, positions, etc. are as of the time of the interview)
Although RestoreVision is a Keio University startup, all of the research in Japan on rhodopsin gene therapy, which we are working to commercialize, can be traced back to Kyoto University. In other words, it is a startup born from the fusion of Keio University and Kyoto University. There are many patients around the world who go blind due to retinal abnormalities. If this gene therapy can save people from blindness caused by retinal abnormalities, it will be a great gospel for society.

Osami Kono

Website of Restore Vision Inc.
Please feel free to contact us if you have any questions or concerns.
Inquiry Form